Our Strategic Clinical Development
NOK Therapeutics is a leader in autologous NK cell therapies, targeting critical unmet needs in oncology. Our pipeline focuses on minimal residual disease (MRD)-positive situations and enhancing antibody-dependent cellular cytotoxicity (ADCC). Explore our innovative strategies and clinical development plans, below.
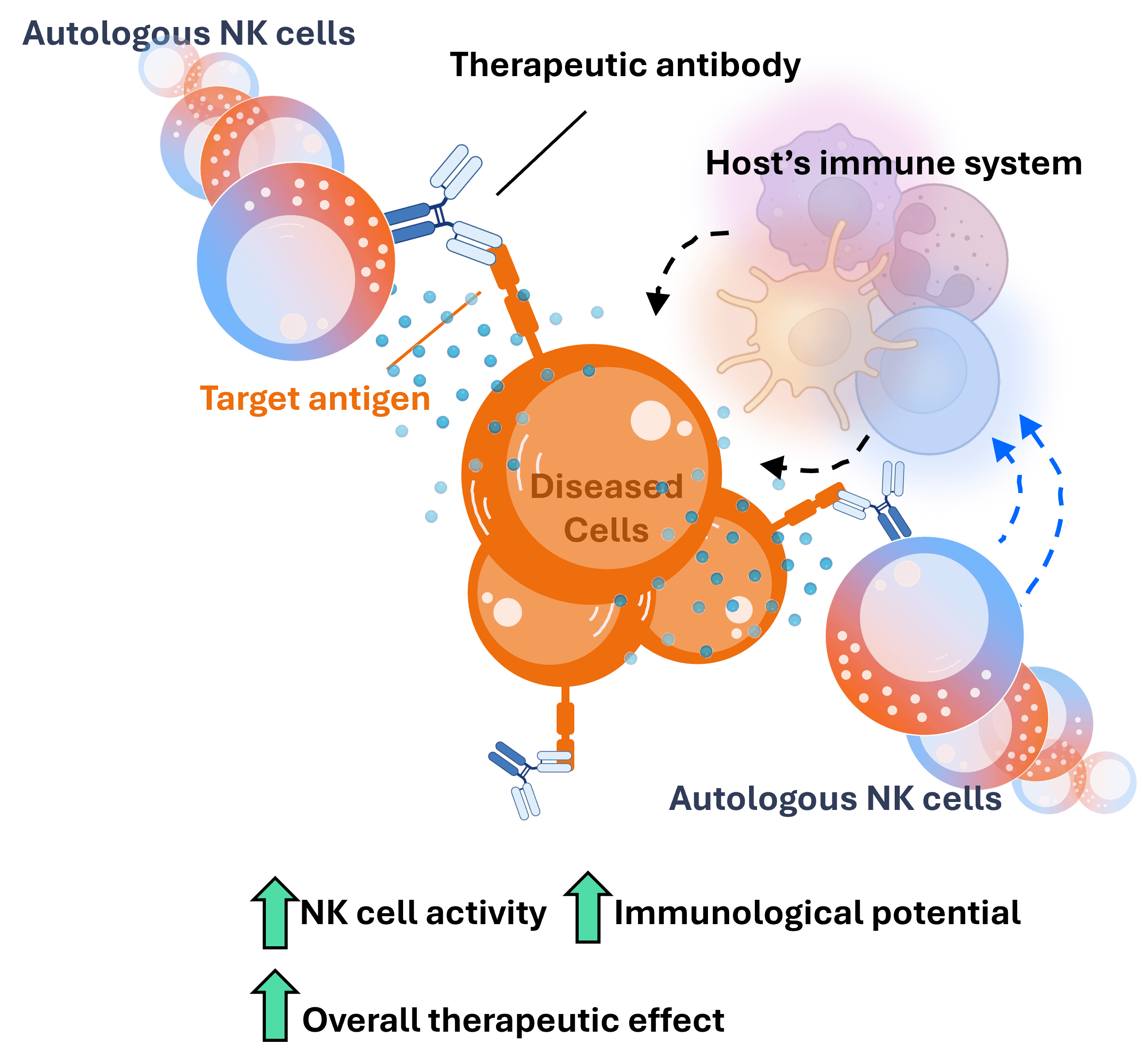
Augmenting Therapeutic Antibodies
Our autologous NK cell therapy provides a robust foundation that empowers therapeutic antibodies to induce a powerful anti-cancer effect.
Clinical Limitation of antibodies: Therapeutic antibodies rely on the activity of immune cells – mainly NK cells and macrophages – to induce their effects. In cancer patients, these two cell populations are often compromised, significantly diminishing the activity of antibodies.
Our Solution: Our autologous NK cell platform overcomes these limitations in a unique, potentially revolutionary manner, and boosts the therapeutic activity of antibodies.
Follow the tabs below to discover exactly what we mean and the clinical implications.
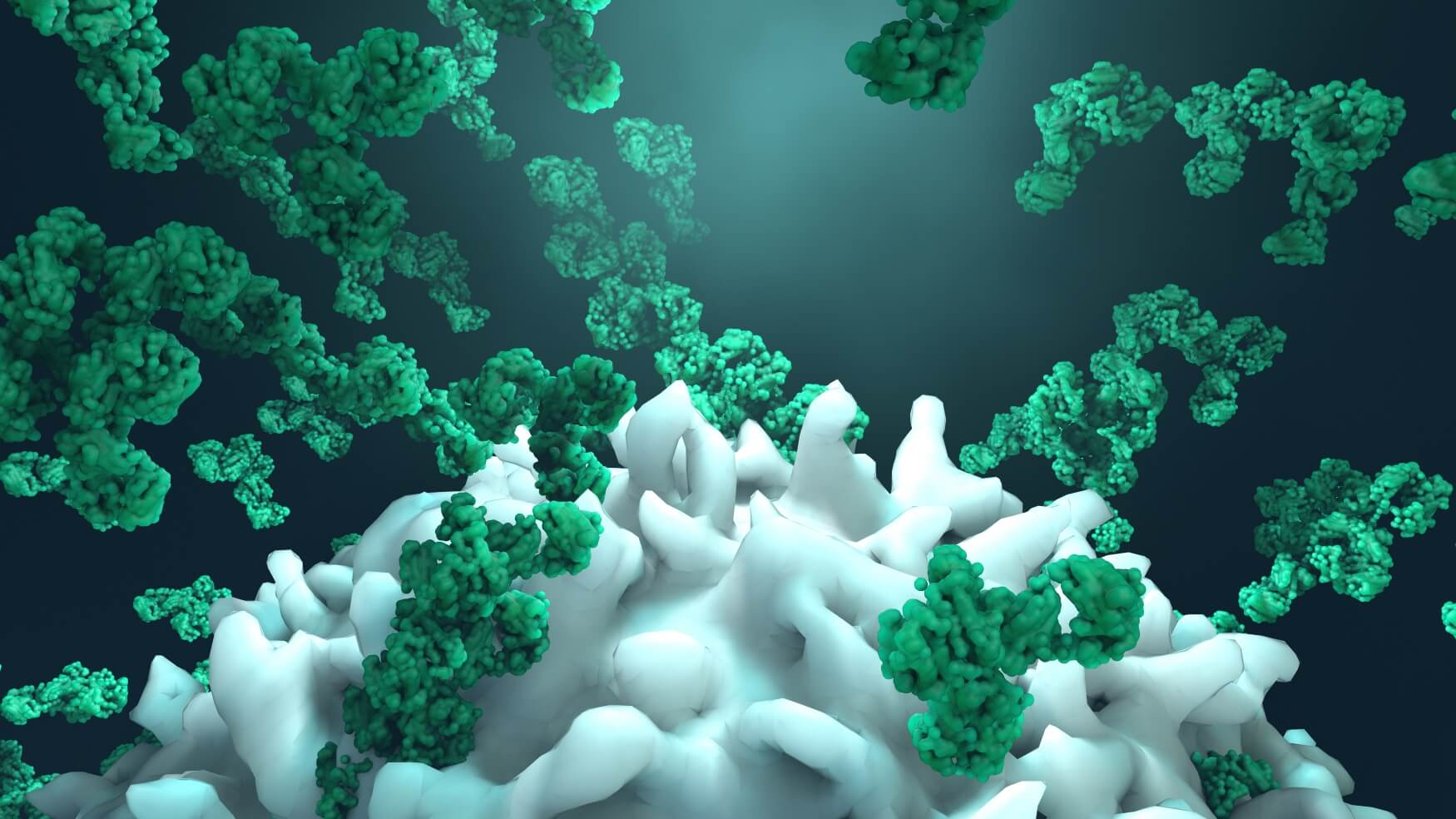
Mechanism of action of mAbs: When a monoclonal therapeutic antibody is administered, it will bind to its target antigen on diseased cells and induce cell death in two main ways: i) inhibition of the target, and ii) recruitment of cytotoxic immune cells, mainly NK cells, that will promptly kill the target cells.
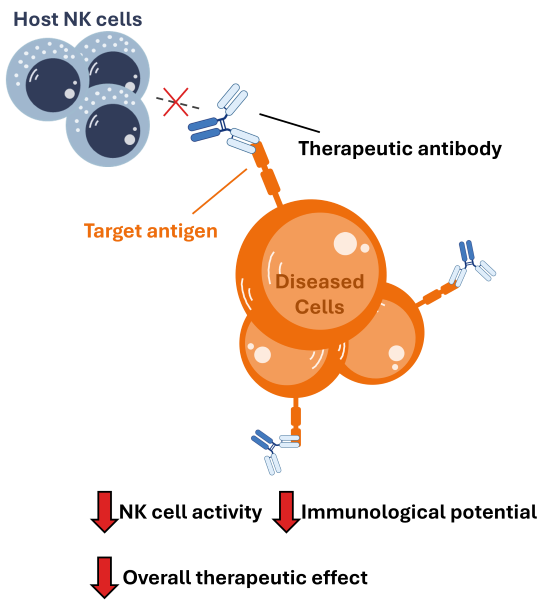
Trouble with cancer patients: In order for cancer to grow, it must learn to suppress and inhibit the activity of the surrounding immune cells. This allows the cancer to grow without being killed by the patient’s immune system.
Clinical Limitation: So, a cancer patient’s NK cells are typically very suppressed. Then, when an antibody is administered, there aren’t very many competent NK cells around to induce the therapeutic effect resulting in poor clinical performance.
NK cell therapies boost NK cell activity: A potential solution to overcome cancer-induced immunosuppression of NK cells and boost antibody activity is to infuse an NK cell therapy. This will ensure that a large population of immunologically activated NK cells are present to interact with the antibody.
Limitation of allogeneic cell therapies: Most NK cell therapies are derived from a healthy donor. Unfortunately, in order to ensure that the donor-derived NK cells aren’t rejected upon infusion, the recipient’s immune system must be depleted. This is typically done by treating the patient with highly toxic therapy that eliminates the majority of their immune cells.
Clinical implications: This harsh treatment can disqualify many patients, limiting the accessibility of such therapies. The other problem is that NK cells do not operate alone – they work to stimulate other immune cells like T cells. Well, since the patient’s immune system has been destroyed, the NK cells are stranded. This can lead to suboptimal therapeutic activity of the antibody.
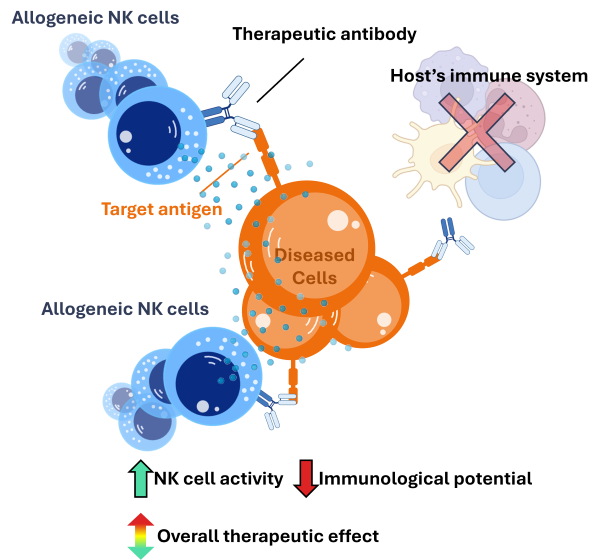
Autologous NK cells offer a more elegant solution: On the other hand, autologous NK cells – derived from the patients themselves – do not require lymphodepletion prior to infusion. This results in two major therapeutic benefits:
Benefit #1: Since lymphodepletion is not necessary prior to infusion, autologous NK cells can be administered with ease, increasing patient access and broadening the scope of the potential impact. This allows for a more flexible and convenient re-dosing with more strategic infusion timelines.
Benefit #2: With an intact immune system, the immunological activity of the NK cells – sparked by the antibody – has the potential to activate other, non-NK, immune cells such as dendritic cells and T cells. This would result in a broader and more robust anti-cancer effect.
Auto-NK in Metastatic Urothelial Carcinoma
+ αPD-L1 mAb Bavencio (avelumab)
We have strong rationale and proof of concept preclinical data to justify the combination of our auto-NK cell platform with Merck’s PD-L1 antibody avelumab in metastatic urothelial cancer. We plan to initiate a phase I trial.
Auto-NK in Merkel Cell Carcinoma
DISCOVERY
PRECLINICAL
PHASE 1
PHASE 2
Minimal Residual Disease Settings
We have strong rationale to focus on patient populations where an allogeneic product would not be feasible – primarily in minimal residual disease (MRD)-positive settings.
Rationale: Patients in MRD-positive settings often have low disease burden but are at high risk of relapses. Since these patients have likely recently already undergone harsh therapeutic regimens, administering a highly toxic lymphodepleting regimen in these patients would not be feasible due to their fragile condition.
Opportunity: Our autologous NK cell therapy provides an ideal solution for these patients. Since our platform does not require lymphodepletion, we can administer the therapy without subjecting patients to additional toxicity. The goal is to “clean up” residual disease post-treatment, potentially resulting in more durable, long-lasting remissions or even cures.
Clinical Potential: By targeting residual malignant cells in MRD-positive settings, our therapy could significantly reduce relapse rates and extend remission periods. This approach could be particularly transformative in diseases like multiple myeloma, where relapses after HSCT are common.

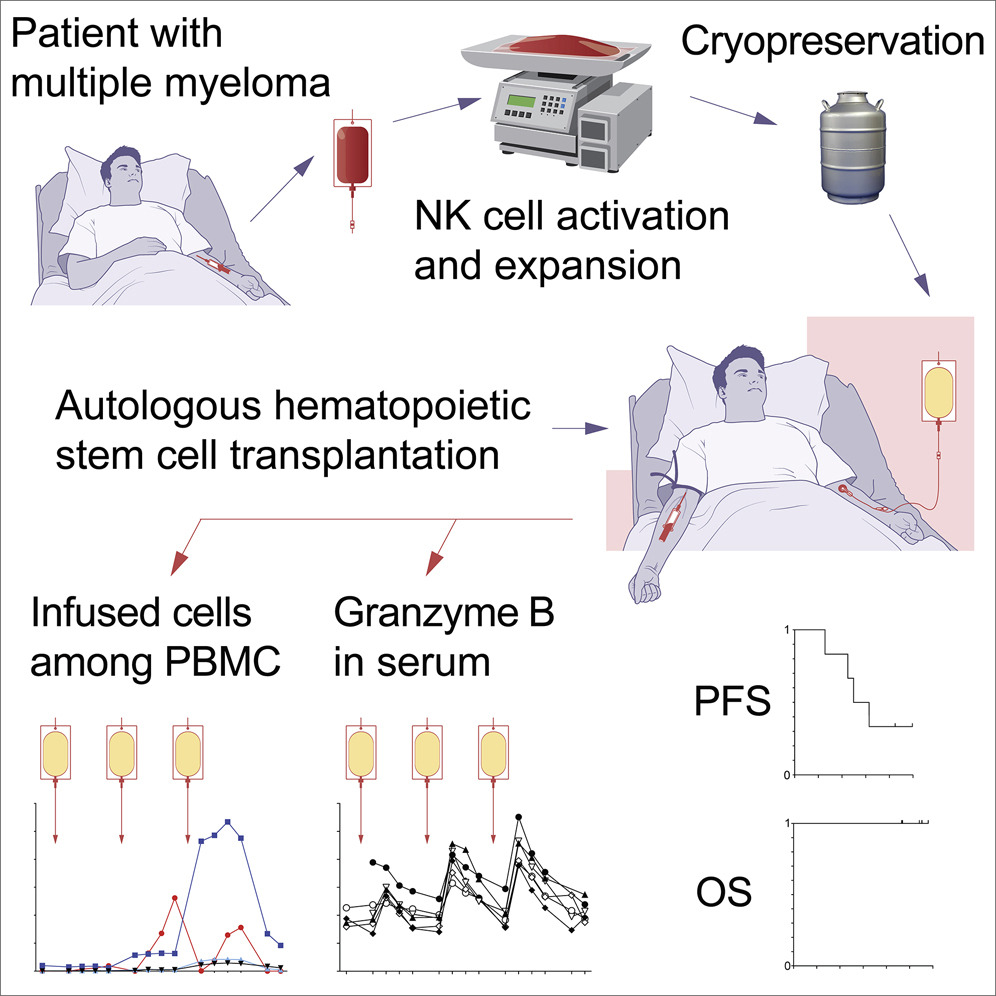
Auto-NK (Post-HSCT Multiple Myeloma)
Building on positive phase I data, we are planning to initiate a phase II study investigating the potential for our autologous NK cell therapy in the post-HSCT setting in Multiple Myeloma.
Become an Investor
Visit out investor hub for company details and more information on how to join our team of investors.
Our Mission
At NOK Therapeutics, we are dedicated to ensuring that more cancer patients can benefit from the life-changing potential of cellular therapies, regardless of their previous treatment history or current health status.